68
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Powder:
Glycine
Sodium chloride
Sodium citrate
Solvent:
Water for injections
6.2 Incompatibilities
Berinert should not be mixed with other medicinal products and
diluents in the syringe/infusion set.
6.3 Shelf life
30 months
After reconstitution, from a microbiological point of view and as
Berinert contains no preservative, the reconstituted product should
be used immediately. The physico-chemical stability has been
demonstrated for 48 hours at room temperature (max. 25°C).
However, if it is not administered immediately, storage shall not
exceed 8 hours at room temperature. The reconstituted product
should only be stored in the
vial.
6.4 Special precautions for storage
Do not store above 25°C.
Do not freeze.
Keep the vial in the outer carton, in order to protect from light.
For storage conditions after reconstitution of the medicinal
product, see section 6.3.
6.5 Nature and contents of container
Powder:
Injection vial of colourless glass Type II, sealed with
bromobutyl rubber infusion stopper Type I, aluminium seal and
plastic flip-off cap.
Solvent:
10 ml water for injections in an injection vial of colourless
glass Type I, sealed with chlorobutyl rubber infusion stopper Type I,
aluminium seal and plastic flip-off cap.
Administration set:
1 filter transfer device 20/20, 1 disposable
10 ml syringe, 1 venipuncture set, 2 alcohol swabs, 1 plaster*
6.6 Special precautions for disposal and other handling
Any unusedmedicinal p roduct orwastematerial shouldbedisposed
of in accordance with local requirements.
Method of administration
General instructions
– The solution should be colourless and clear. After filtering/
withdrawal (see below) reconstituted product should be
inspected visually for particulate matter and discoloration prior
to administration.
– Do not use solutions that are cloudy or have deposits.
– Reconstitution andwithdrawal must be carriedout under aseptic
conditions. Use the syringe provided with the product.
Reconstitution
Bring the solvent to room temperature. Ensure product and solvent
vial flip caps are removed and the stoppers are treated with an
aseptic solution and allowed to dry prior to opening the Mix2Vial
package.
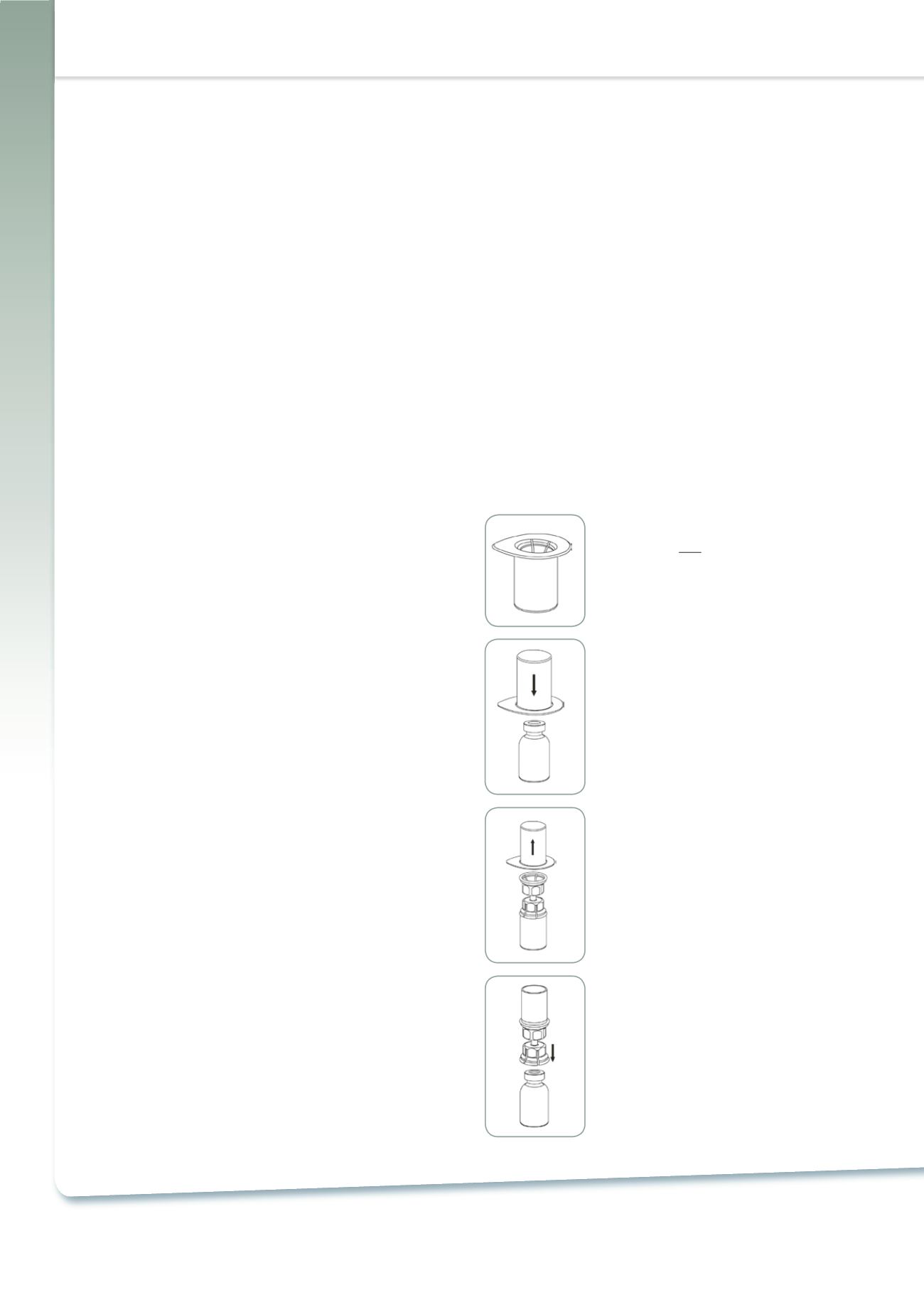
1. Open the Mix2Vial package by peeling
off the lid. Do
not
remove the Mix2Vial
from the blister package!
2. Place the solvent vial on an even, clean
surface and hold the vial tight. Take the
Mix2Vial together with the blister package
and push the spike of the blue adapter end
straight down
through the solvent vial
stopper.
3. Carefully remove the blister package
from the Mix2Vial set by holding at the rim,
and pulling
vertically
upwards. Make sure
that you only pull away the blister package
and not the Mix2Vial set.
4. Place the product vial on an even and
firm surface. Invert the solvent vial with the
Mix2Vial set attached and push the spike of
the transparent adapter end
straight
down
through the product vial stopper.
The solvent will automatically flow into the
product vial.
*The administration set and Mix2Vial™ are supplied in most countries.


