14
2.2 Characterizing the Epidemiology of HAE
2.21 Identifying the Genetic Etiology of HAE
C1-INH is the primary control protein that regulates the
activation of mediators of vascular permeability, including
bradykinin.
7,12
The gene for C1-INH, aptly named
C1NH
or
SERPING1
, is located on chromosome 11, specifically
11q12-q13.
5,13,14
SERPING1
appears to be highly susceptible
to mutation.
13
To date, 243 distinct mutations of
SERPING1
have been identified as causes of HAE.
15
A single mutation
can cause HAE as a dominant trait. In nearly 25% of
diagnosed HAE patients, these mutations occur
de novo
.
16,17
By definition, patients with a
de novo
mutation will have
a negative family history. In the remaining 75% of HAE
patients, the condition is inherited as an autosomal
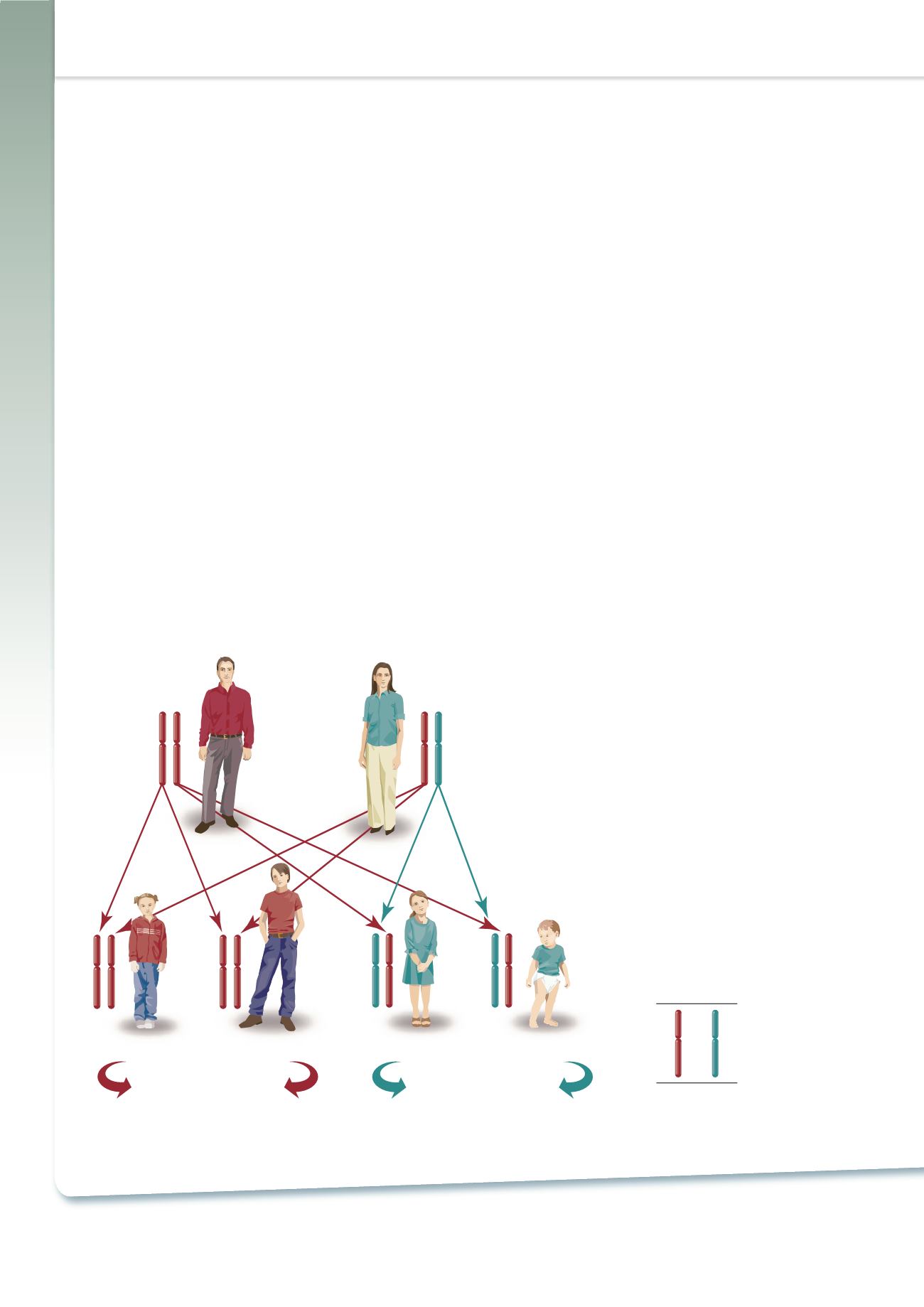
dominant trait (Figure 4).
5-10
As a result of the gene defect,
individuals with HAE produce either a deficient amount of
C1-INH and/or dysfunctional C1-INH.
5,18
Unaffected
Father
Affected
Mother
Unaffected
Child
Unaffected
Child
No Progeny
With HAE
50-50 Risk (2 in 4)
of Passing HAE Gene
Affected
Child
Affected
Child
LEGEND
Healthy
Gene
HAE
Gene
Unaffected
Father
Affected
Mother
LEGEND
Healthy
Gene
HAE
Gene
Figure 4 – Hereditary Angioedema is
an Autosomal Dominant Disease
2.22 Estimating the Incidence and Prevalence
of HAE
The exact incidence and prevalence of HAE is not known.
It is estimated that this rare disease occurs in only 1:10,000
to 1:150,000 individuals and most sources place the
estimate at 1:50,000.
6-8,17,19-21
The disease is not known to
discriminate across racial or ethnic groups.
8,17
As an autosomal dominant trait, the genotype occurs
equally often in males and females. However, it appears
that some women may be more prone to attacks of HAE,
in part, because of shifting estrogen levels that are a normal
part of the menstrual cycle.
5,10,22,23
Individuals with HAE will have the disease genotype for life.
However, the expression of the clinical symptoms varies
widely – even in a single individual at different times and
in different family members within an affected family.
5,6,17
Consequently, some individuals endure several attacks
weekly, while others may be attack-free for months or even
years.
5,17,23
Intraindividual differences are also common,
and patients may experience numerous attacks during
one period of life, and be virtually symptom-free another.
5
Approximately 85%of patients with HAE due to functional
C1-INH deficiency have type I HAE (HAE-I), i.e., they
synthesize inadequate amounts of C1-INH.
5,18
The remainder
have type II HAE (HAE-II), i.e., they produce functionally
inactive C1-INH.
5,18


